Crimean-Congo Haemorrhagic Fever
Crimean-Congo haemorrhagic fever (CCHF) is a severe form of haemorrhagic fever caused by a virus of the genus Nairovirus. The first cases were documented in 1944, when Soviet forces clashed with the German army in Crimea, but there are also descriptions of a probable, similar disease in Tajikistan that date back to the 12th-century.
The virus was not identified until 1967, when Soviet physician Mikhail Chumakov (responsible, among other things, for the discovery of the tick-borne encephalitis virus) isolated it in an Uzbek patient. In parallel, a virus responsible for a very similar disease had already been isolated in 1956, in the Democratic Republic of Congo. Confirmation of the similarity between the two viruses came in 1969, hence giving the disease its present name.
CAUSES
CCHF virus belongs to the genus Nairovirus, of the order Bunyaviridae. The genus Nairovirus includes 34 different viruses, divided into 7 distinct serological subgroups, 3 of which can cause disease in humans.
The nairovirus genome is structured into 3 single-stranded RNA segments, named S, M and L, according to their respective sizes. The virion consists of a lipid envelope and its diameter measures about 90 to 120 nm.
Today the viral RNA (particularly the S segment) has been sequenced and eight genetic lines have been identified.
- A European strain, which includes the viruses found in Eastern Europe, that covers a region between the Balkan Peninsula and Russia, passing through Turkey.
- The AP92 strain, identified mainly in Greece, is part of an independent group and is probably less pathogenic.
- A strain found in Central Asia (Kazakhstan, Tajikistan, Uzbekistan and China)
- The Pakistan/Madagascar strain, plus a few detected in Iran.
- A strain that consists of the remaining strains found in Iran, together with those from Senegal and Mauritania.
- The other three lineages are only found in Africa.
CCHF virus has been found in about 30 different species of ticks. The most efficient vector belongs to the genus Ixodes, the tick Hyalomma marginatum, which can contract the virus by feeding on an infected host.
The virus replicates within the tick’s organism, particularly in the cells of the intestinal wall, and spreads to various other tissue, with the highest concentrations found in the salivary glands and reproductive organs.
Vertebrates are the hosts that contribute most to the spread of CCHF. Surveys of seropositivity rates have shown that large herbivores are more frequently infected. Birds, on the other hand, do not develop the virus, with the exception of ostriches, although they can help spread the disease by carrying CCHF-infected ticks for hundreds of kilometres.
TRANSMISSION
Human infection usually occurs through contact with infected blood from a tick (either via a direct bite or by squashing an infected tick without wearing gloves).
Cases increase during the spring and summer, when adult ticks feed to complete their life cycle, especially if the previous winter was mild, which favours the survival of these arachnids.
The ability of ticks to transmit CCHF virus to humans also depends on the ecosystem in which they develop. In regions with large numbers of both small mammals, such as hares and hedgehogs, and large mammals such as cattle and sheep, the virus seems to circulate silently with only a few sporadic "incidental" cases in humans.
Conversely, as appears to have been the case in Crimea in 1944, when wild hares proliferated on the farms abandoned during the German occupation, and the number of farm animals decreased significantly, soldiers and laborers repopulating the area indirectly represented a new source of blood for adult Hyalomma ticks, which resulted in a high incidence of CCHF cases in humans.
Humans can also be infected by direct contact with animal blood or other infected tissues, although muscle acidification after the death of the animal deactivates the virus.
In a study describing a series of 1,820 cases recorded in Turkey between 2002 and 2007, 62 percent of patients reported close contact with animals. Nearly 90 percent of reported cases occurred among farmers, slaughterhouse workers, or butchers. It has also been shown that the seropositivity rate is higher in the aforementioned categories, as well as elderly patients and those of lower socioeconomic status.
Human cases are predominantly observed in males, a fact strongly correlated with occupation in at-risk countries, where agricultural activities are mostly performed by men.
The possible sexual transmission of CCHF should also be considered, although very few cases have been documented. However, several cases of mother-to-child transmission have been reported, with severe haemorrhagic syndromes in new-borns and subsequent death. However, not all new-born babies are infected, which suggests that the virus is not always transmitted vertically.
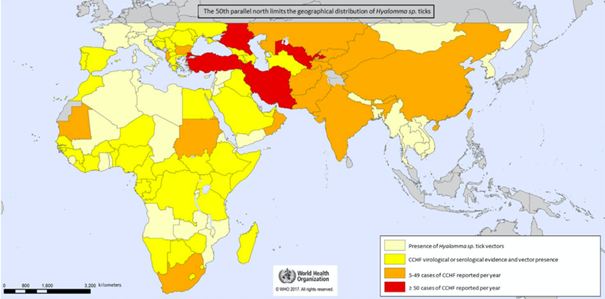
GEOGRAPHICAL DISTRIBUTION
CCHF has the widest geographical distribution of all tick-borne viruses that affect human health and the second largest geographical distribution of all arboviruses after the Dengue virus.
During the 20th century, major outbreaks of CCHF occurred in the former Soviet Union (e.g., Crimea, Rostov, Astrakhan); Bulgaria with 1,105 cases reported between 1953 and 1974, and China, where 260 farmers were infected between 1965 and 1994 in the Xinjiang region, with a reported mortality rate of 80% (probably skewed because only the most severe cases were recorded).
Currently, the main outbreaks are in the Middle East (Turkey and Iran), with more than 10,000 cases documented in Turkey since 2002.
Numerous studies have been conducted to determine the rate of seropositivity and circumscribe the geographical area affected by the virus. A large number of seropositive cases have been found in ruminants (sheep, cows, goats), amplifying hosts of CCHF, which serve as reliable indicators of the presence of the virus in certain regions.
There is a logical relationship between a high rate of seropositivity in animals in a given region and the incidence of the disease in humans. In fact, in a study of 1,165 ruminants in Bulgaria and Turkey, seropositivity rates of 26 percent and 57 percent, respectively, were reported, with significant divergences from region to region.
In Africa, although fewer human cases of CCHF are reported than in the Middle East, seropositivity rates are sometimes high among livestock, with 1.6% in the Democratic Republic of Congo, 66% in Mali, 67% in Mauritania, and 21% in dromedaries in Sudan.
In Europe, infection rates are lower than in the aforementioned African regions. However, the disease has been identified in livestock in Bulgaria, Hungary, Albania, Kosovo and Greece, and even in two bats in southern France.
In 2002, the virus was also detected for the first time in ticks sampled from deer in Spain. The strain of origin was the African strain, which is phylogenetically related to the strain found in ticks that infest migratory birds in Morocco. To date, however, very few ticks in Spain have been found to carry the virus (between 0 and 3.2%).
SYMPTOMS
The very high seropositivity rates found in some endemic areas (as high as 27% in some regions of Romania and Greece), suggest that a great many CCHF cases are asymptomatic or cause such limited symptoms that patients do not seek medical treatment. In fact, an estimated 88% of HIV-positive patients in Turkey experienced only limited symptoms.
In symptomatic cases, the average incubation period is 2 to 7 days. Onset is sudden, with fever, muscle pain, dizziness, neck pain and stiffness, back pain, headache, burning eyes, and photophobia (sensitivity to light). Patients may also experience nausea, vomiting, diarrhoea, abdominal pain, and sore throat in the initial stages, followed by mood swings and confusion.
After about 4 to 5 days, the hemorrhagic phase begins, characterised by bleeding of the mucous membranes: epistaxis, hematemesis, more rarely melena (blood in stools), hemoptysis (coughing of blood), and hematuria (blood in urine), but bleeding of the skin (ecchymosis/purpura) is also often observed.
The convalescence stage begins 10-20 days after the onset of the first clinical symptoms and lasts for approximately 10 days. Patients experience marked fatigue, tachycardia with labile blood pressure, temporary alopecia, and memory impairment.
DIAGNOSIS
Given the nonspecificity of symptoms, early diagnosis is difficult, as hemorrhagic manifestations are also characteristic of other infectious diseases in the same geographical areas.
Certain determining factors must inevitably be taken into consideration, such as:
- The presence of tick bite(s);
- Contact with livestock;
- Employment in certain occupational categories in endemic area;
- Interhuman contact with CCHF cases (also for occupational reasons), particularly during established or suspected outbreaks.
Virological diagnosis of CCHF relies on serology and virus detection. Antibodies (detected by ELISA, immunofluorescence, and neutralisation methods) appear 5-7 days after disease onset; in the initial stage, molecular techniques are the most useful for diagnosis. However, specific IgG antibodies can be detected for at least 5 years.
Consequently, an acute-stage infection can be identified by detecting IgM (but not IgG) in an initial serum sample (i.e. IgM and IgG simultaneously), or seroconversion with a fourfold increase in antibody titers between two subsequent serum samples. The specificity of serological testing is excellent, with no false positives or cross-reactivity with other viruses reported.
TREATMENT
There is no specific drug therapy for CCHF. Treatment of patients with CCHF is based on symptomatic treatment to manage electrolyte imbalances caused by gastrointestinal disorders, hemodynamic support (with fresh frozen plasma or platelet transfusion), transfusion of cell concentrates.
Based on encouraging data, some authors recommend treatment with ribavirin, which appears to be able to reduce the mortality rate, but only if administered in the early stage of disease. Post-exposure prophylaxis also appears to reduce the risk of infection.
PREVENTION
It is important for people travelling to endemic areas to be adequately informed about vector control measures against tick-borne diseases (e.g. insect repellents, wearing long clothing), the need to wear gloves when removing ticks, and the risks of contamination due to meat processing practices and contact with livestock. In any case, the risk of contracting the disease while travelling to an endemic area, then re-importing it back to the traveller’s country of origin, is considered very low.
With regard to a vaccine, animal studies have led to divergent results. One team was able to trigger both a cell-mediated and humoral immune response in mice using a vaccine strain, a reaction that did not, however, reduce mortality rates.
Stage 1 vaccine trials are currently underway in humans, but the antibodies produced appear to have only a limited ability to neutralise the virus.
Bibliography:
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 2013;100(1):159–89.
- Papa A, Weber F, Hewson R, Weidmann M, Koksal I, Korukluoglu G, et al. Meet- ing report: First International Conference on Crimean-Congo hemorrhagic fever. Antiviral Res 2015;120:57–65.
- Spengler JR, Bente DA, Bray M, Burt F, Hewson R, Korukluoglu G, et al. Second International Conference on Crimean-Congo Hemorrhagic Fever. Antiviral Res 2018;150:137–47.
P. Fillâtre, M. Revest, P. Tattevin. Crimean-Congo hemorrhagic fever: An update. Fièvre hémorragique de Crimée-Congo: mise au point. 0399-077X 2019 Elsevier.




